 Frenchay Dysarthria Assessment 2 (FDA 2): Scoring | Interpretation:
Frenchay Dysarthria Assessment 2 (FDA 2): Scoring | Interpretation:
The Frenchay Dysarthria Assessment (FDA) represents a major step forward in the clinical approach to dysarthria, one of the most common types of acquired speech disorders, which results from neuromuscular disorders. As methods are becoming sophisticated in evaluating neuromotor activity using technology, the field has struggled with non-standardized and non-replicable clinical tools in the treatment environment. Developed at Frenchay Hospital, the FDA is a standardized test providing a definitive diagnosis of dysarthria by speech pathologists. The FDA’s Rare Disease and Novel Drug Development program has responded to this by recommending an effective and accessible protocol that meets the growing need to assess and treat the communication form commonly associated with the neurodegeneration of dysarthria — speech therapy for dysarthria adapted from international guidelines a proven therapy that has been successfully applied to over 600 patients with the help of a network of over 200 speech therapists.
This article explains what the FDA 2 is, its protocol, categories, scoring protocol, and other equipment needed to implement it. Comprehension of the FDA 2 is indispensable for speech pathologists but also for interdisciplinary clinical teams working with the diagnosis and management of patients presenting with dysarthric symptomatology.
- Author: Pamela M Enderby
- Publication Date: 2008
- Age Range: 12 years to adult
- Administration Time: 20 to 40 Minutes
What is Frenchay Dysarthria Assessment (FDA)?
Dysarthria refers to the speech abnormalities which occur as a result of neuromuscular disorders. Dysarthria is among the most common types of acquired speech disorder but with little progress regarding its clinical management. While there is a range of robust technical methods to quantify neuromotor activity, there is no easily replicable clinical tool to use in the treatment context. A standardized test, called the Dysarthria Assessment, developed at Frenchay Hospital, allows speech pathologists to accurately diagnose dysarthria. More than 600 patients have undergone this test, performed by more than 200 speech therapists.
Protocol for Frenchay Dysarthria Assessment 2 (FDA)
Taking into account that the protocol for a dysarthria test should yield results that are directly implementable in therapy, Dysarthria is frequently speech that is confusing on the part of speech therapists, therefore, it is important to describe how affected areas of function relate to one another in comparison to intact areas of function. It has to do with a total approach that is needed to find a relevant approach to treatment. Further testing procedures, therefore, need to be able to detect speech differences and changes, in order to know if our treatment procedures are appropriate or not in terms of specific disorders. Audio tapes can provide recorded samples which exacerbate this lack of understanding, however, they have limited utility when it comes to interpreting dysarthric speech.
The test is also highly efficient and user-friendly. Test formats should be clear, practical, and discouraging to therapists from modifying procedures to speed the administration (consideration should be given to patients with poor stamina).
A reliable test is all about standardization. In a standardized administration, one can assess test-retest reliability with intra/inter-judge reliabilities, obtain normative data, and of course can compare to specific abnormal groups. Other methods for assessing dysarthria may exist, but these methods are not standardized and remain clinical checklists, limiting their application.
With high reliability, little training should be needed. Eliminate excessive training time for speech pathologists by paying attention to the test procedure and the scoring system. Long or costly training could signal a design flaw in the test, which impacts the reliability of administration and scoring.
It should be clear and, especially if the speech pathologist is involved in a clinical team, include other professions. In addition, patients displaying dysarthric symptomatology may assist in the identification or confirmation of a medical diagnosis.
Equipments for Administer FDA-2
1. Test manual
2. Scoring graph
3. Tongue depressor
4. Stopwatch
5. Tape recorder
6. Glass of water
7. Word cards
Frenchay Dysarthria Assessment 2 (FDA 2) Subtests
The examination is segmented into 8 primary subtests:
- Reflex
- Respiration
- Lips
- Jaw
- Palate
- Laryngeal
- Tongue
- Intelligibility
Influencing factors of learning
- Sight
- Teeth
- Language
- Mood
- Posture
- Rate and Sensation
There are subtests that make up each major category. Testing each section of a chart is recommended, and although it is not mandatory, you should stick to the order of the chart as closely as possible. Evaluate each subtest within the section in the order indicated. Follow the process, and identify which grade best describes this patient’s conduct. Once you know the test, the assessment becomes possible in an informal interview style.
Frenchay Dysarthria Assessment 2 (FDA 2) Scoring
Some definitions describe grades in terms of ranges of behavior. There is no requirement for the patient to demonstrate every behavior present for a level in order to be awarded a given grade. For example, when a patient states that he/she has to take a longer time to eat because of the fear of coughing even without coughing, he/she belongs to a certain grade. Likewise, a person who chokes from time to time but is not aware or indifferent about it would score within that grade.
By specifying degrees of difficulty (less than 0 or more than 3 or more than 4.5 or more than 5) the assessor can score on the “in-between” lines if the patient’s behavior does not exactly fit into one of the plates. If the item performance (for example the item performance for item 5) is just worse than 1 but better than 2, simply mark the line between these two grades.
The therapist must examine each component in isolation to evaluate their relative abilities and disabilities. Speech is a concerted system, and the impairment of one part can lead to the dysfunction of another’s behavior. In spite of this difficulty it is the assessor’s task to look at each area by itself, not letting abnormalities in other areas confuse him. Following the test procedure will add to the awareness.
Important: The score should be assigned on the basis of behavior shown by the tester on the 2nd time execution of the specific tasks. The first attempt is for practice and if the patient is attempting the task for a third time, it must not affect the scoring. When the score is obtained, then you will use a ballpoint pen to trace a thick line above the printed line in that place of the graph. The matching on the graph does not have to be completed until the pearl’s assessment has been carried out.
Frenchay Dysarthria Assessment 2 (FDA 2) Interpretation
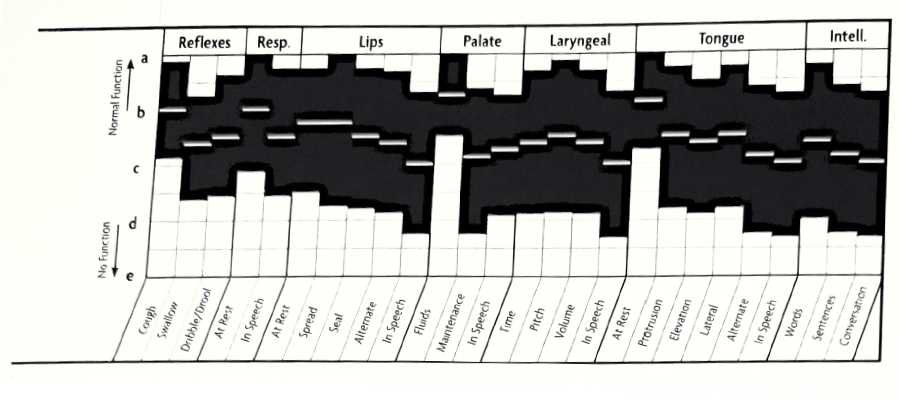
Upper Motor Neuron Lesions FDA Graph
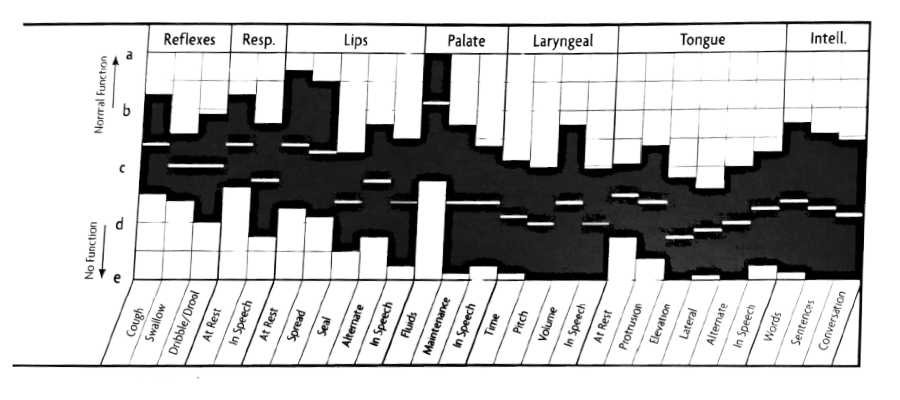
Mixed Upper and Lower Motor Neuron Lesions FDA Graph
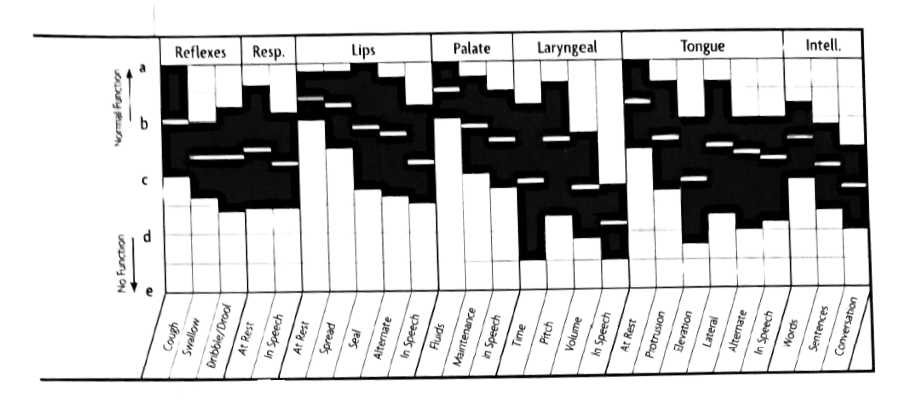
Extrapyramidal Lesions FDA Graph
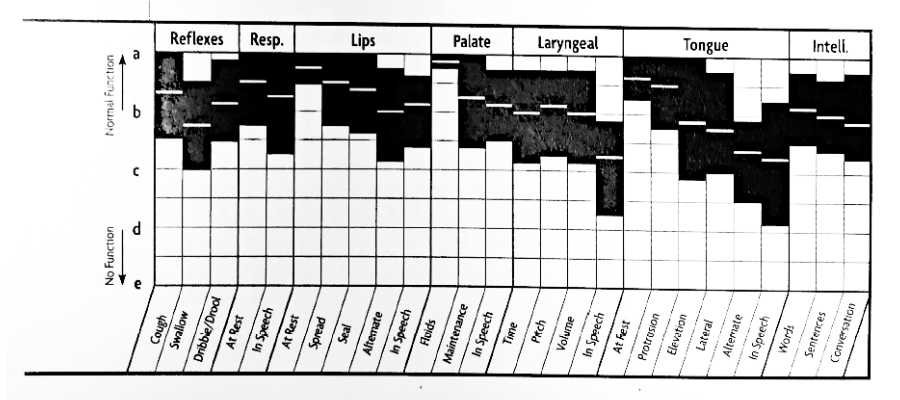
Cerebellar Lesions FDA Graph
Conclusion
The FDA and its updated version, the FDA 2, remain key instruments for detecting and managing dysarthria associated with neuromuscular disorders. These tests have been developed specifically for use in patients with dysphasia using over 600 patients and a large number of speech therapists in the UK and have been validated at Frenchay Hospital. FDA 2 protocol focuses on the efficiency of the test, ease of use, standardization, and reliability of the test results. By outlining specific categories and any above-mentioned influencing factors, a comprehensive overview of how the patient speaks is presented which can help formulate treatment plans that target the individual. The scoring system is simple to administer and interpret, as is the recommended equipment. The FDA 2 results in an excellent tool to facilitate and encourage collaboration between Speech pathologists and clinical teams in a multi-faceted manner to support the patient.
FAQs about Frenchay Dysarthria Assessment 2 (FDA 2): Scoring | Interpretation
1. What is the Frenchay Dysarthria Assessment?
The Frenchay dysarthria assessment (FDA) is a structured examination of the speech of individuals with dysarthria, a speech disorder due to disorders of the neuromuscular system. Designed at Frenchay Hospital, this clinical tool is a comprehensive assessment tool used extensively by speech pathologists to assess and diagnose dysarthria in clients and provide a systematic way to plan treatment.
2. How to score Frenchay Dysarthria Assessment?
As part of scoring the Frenchay Assessment, the patient’s performance is judged in eight main categories; reflex, respiration, lips, jaw, palate, laryngeal, tongue, and intelligibility. The assessment includes a graph with scoring and grades as well as behavioral descriptions for each. Overstay also enables assessors to assess the patient for the proper behavioral performance of a second attempt of specified tasks without influence from the first or subsequent attempts. In parses in between where behavior doesn’t line up exactly with a grade, assessors should be able to express the degree of difficulty using “in-between” lines.
3. Is the Frenchay Dysarthria Assessment standardized?
The Frenchay Dysarthria Assessment is indeed a standardised assessment. This rigidity is important — it increases the reliability of the test through test-retest and intra/inter-judge reliabilities. Genomic measures, such as genetic risk scores, allow the acquisition of normative data that is meaningful against certain abnormal groups. Having a standardized format ensures the results are consistent, making it an effective tool for the identification of dysarthria. Methods of assessment other than ACR/EULAR CR are less well standardized and may play a more checklist like function, reducing their relevance for clinical practice.
4. What equipments are needed to administer FDA-2?
Materials and Equipment The equipment used to administer the Frenchay Dysarthria Assessment 2 (FDA 2) are a test manual, a scoring graph, a tongue depressor, a stopwatch, a tape recorder, a glass of water, and word cards. These tools are crucial to performing a thorough assessment of the reflex, respiration, lips, jaw, palate, laryngeal, tongue, and intelligibility of the patient.
5. What are the influencing factors of learning in the FDA 2 assessment?
Sight, teeth, language, mood, posture, rate, and sensation are some of the factors that affect the performance of the patient in the Frenchay Dysarthria Assessment 2. Overall, these considerations are used during the assessment to give a more complete picture of the patient’s speech abilities and to help create a personalized treatment plan. These factors influencing the assessment should be understood by speech pathologists performing the assessment for the correct interpretation of results.






Thank u for what all u have sent till now .